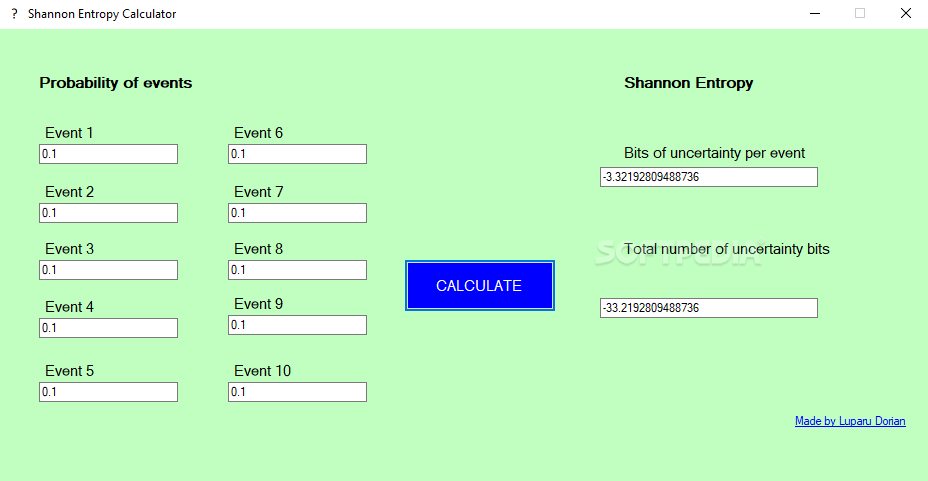
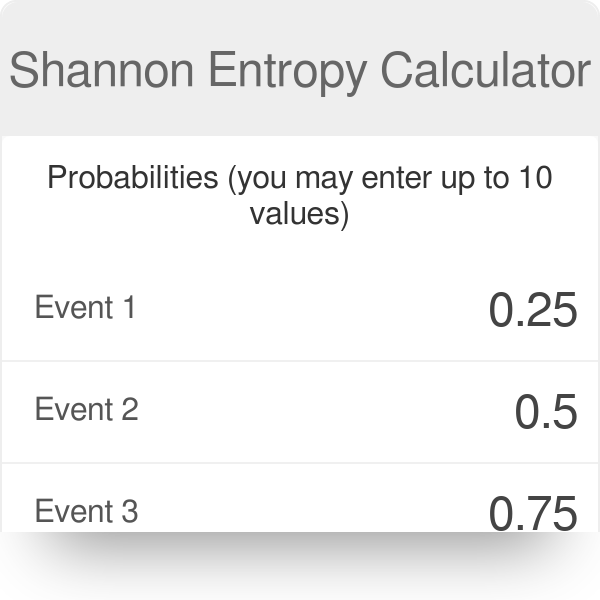
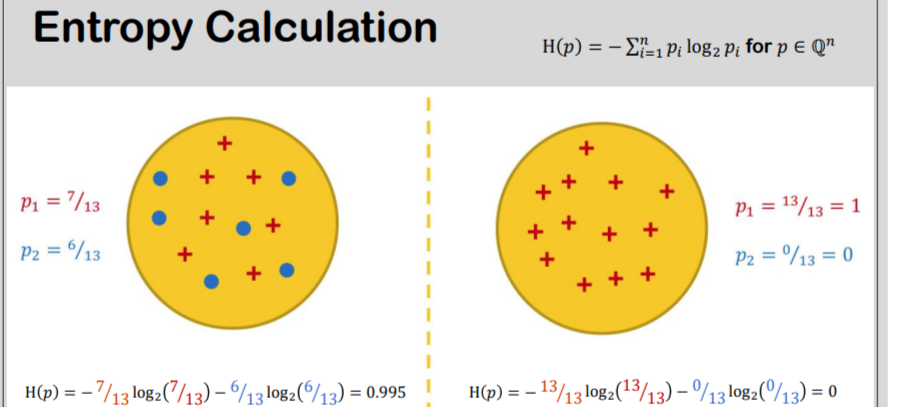
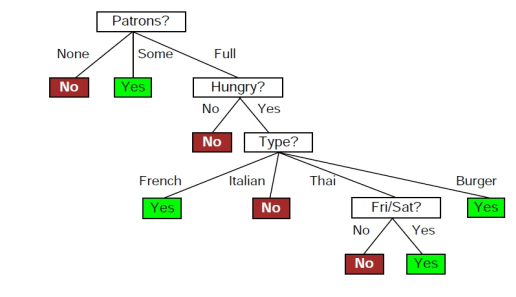
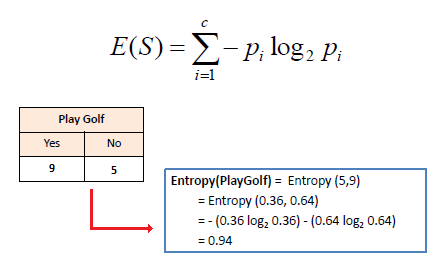
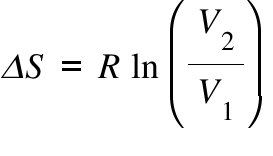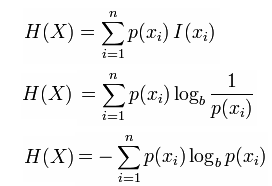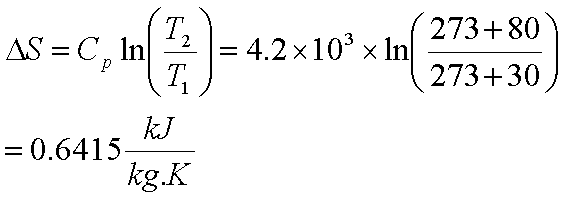How to Build Decision Tree for Classification - (Step by Step Using Entropy and Gain) - The Genius Blog

Example of Shannon's entropy calculation for a gene with four splicing... | Download Scientific Diagram
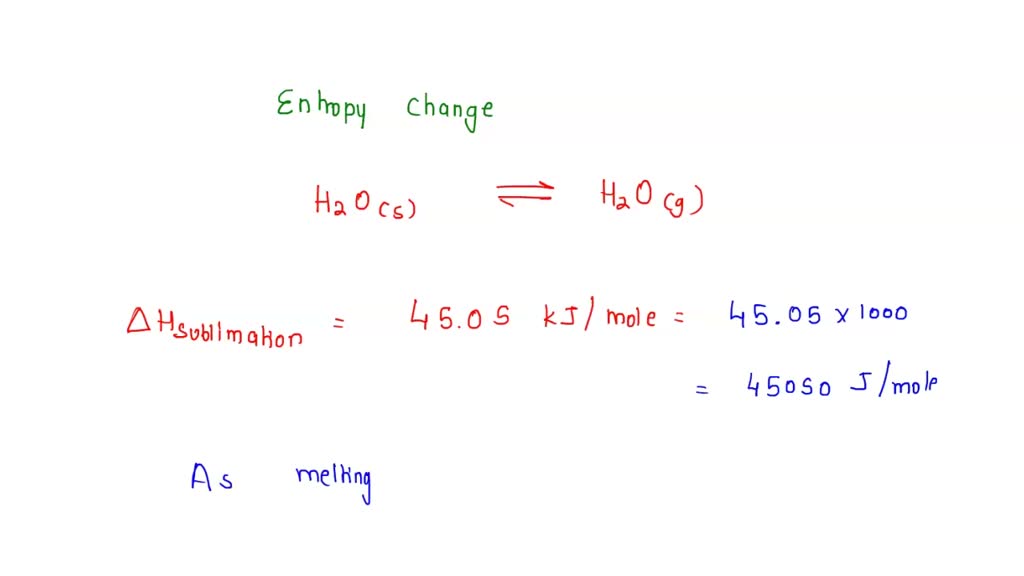
SOLVED: Calculate the entropy change of sublimation of ice, H2O(s) to H2O(g) at the temperature of the triple point of water, 273.16 K. Use the necessary data from the Resource Section.

Entropy Calculation, Information Gain & Decision Tree Learning | by Badiuzzaman Pranto | Analytics Vidhya | Medium
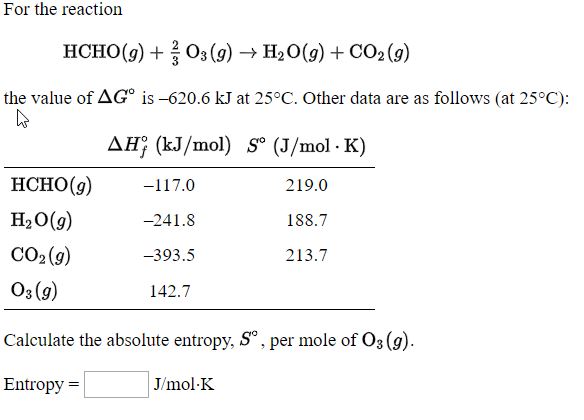
![Using some or all of the information below, calculate the standard molar entropy of I2 at 450 K. S^o = [{Blank}] J/K.mol at 450 K. | Homework.Study.com Using some or all of the information below, calculate the standard molar entropy of I2 at 450 K. S^o = [{Blank}] J/K.mol at 450 K. | Homework.Study.com](https://homework.study.com/cimages/multimages/16/screen_shot_2020-12-02_at_3.01.47_am7814899012014415578.png)
Using some or all of the information below, calculate the standard molar entropy of I2 at 450 K. S^o = [{Blank}] J/K.mol at 450 K. | Homework.Study.com

information theory - How to calculate conditional entropy using using this tabular probability distribution? - Mathematics Stack Exchange


![15.2 Calculate the standard entropy change for a reaction [HL IB Chemistry] - YouTube 15.2 Calculate the standard entropy change for a reaction [HL IB Chemistry] - YouTube](https://i.ytimg.com/vi/IwRy4iYVQLI/maxresdefault.jpg)