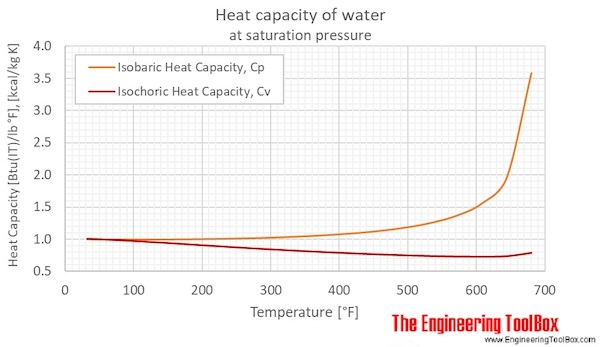
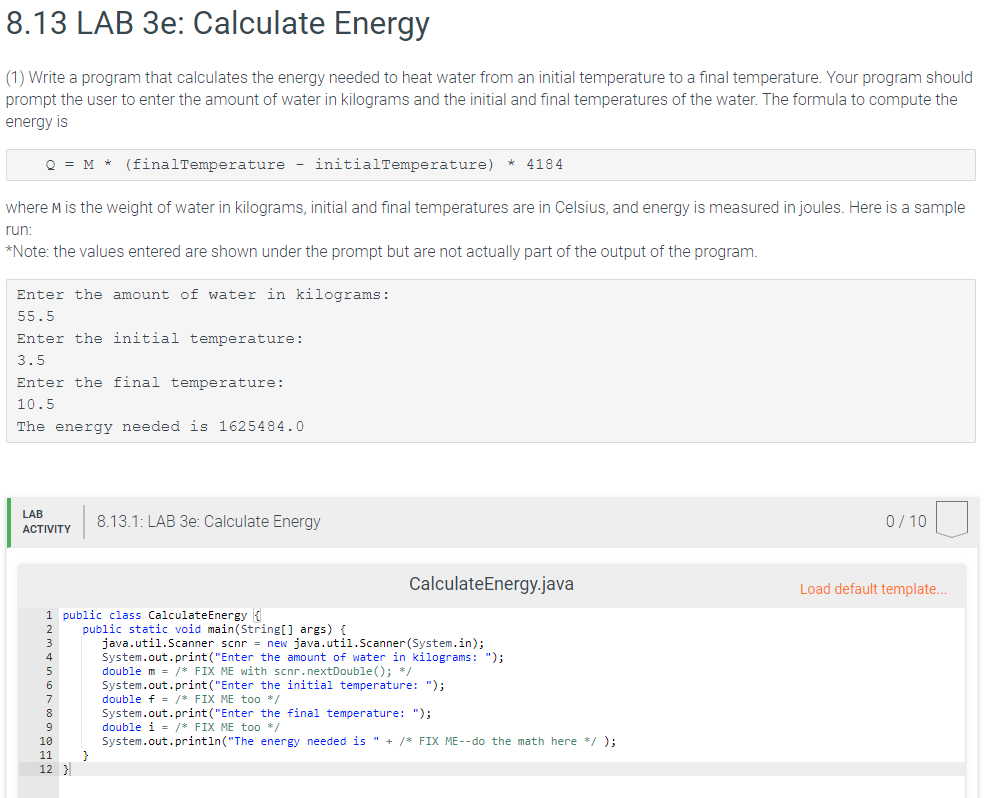
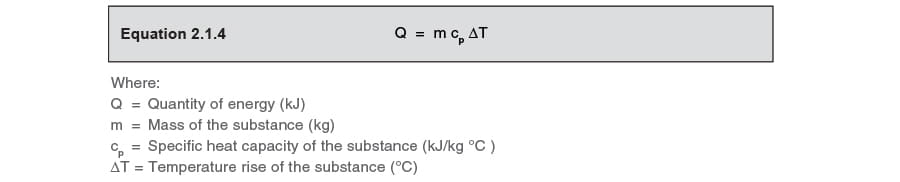
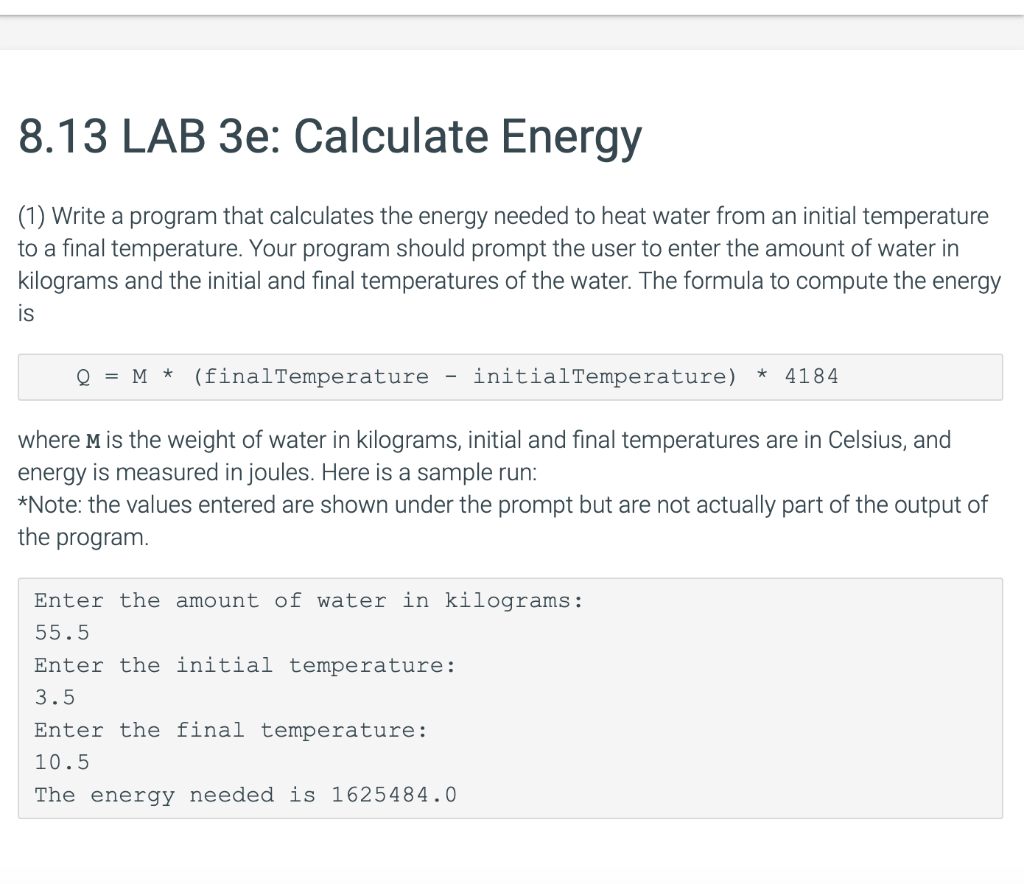
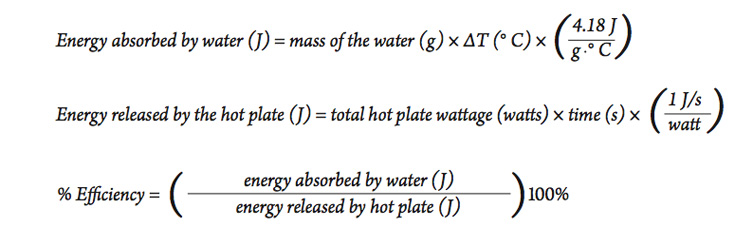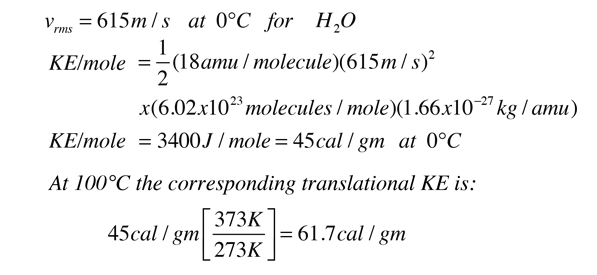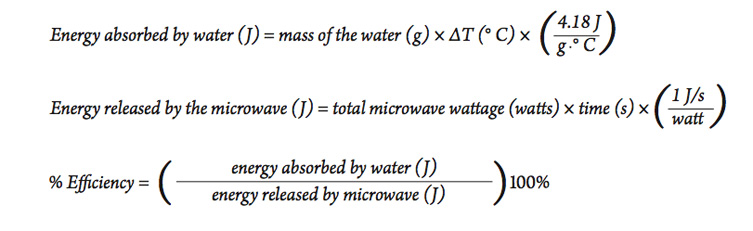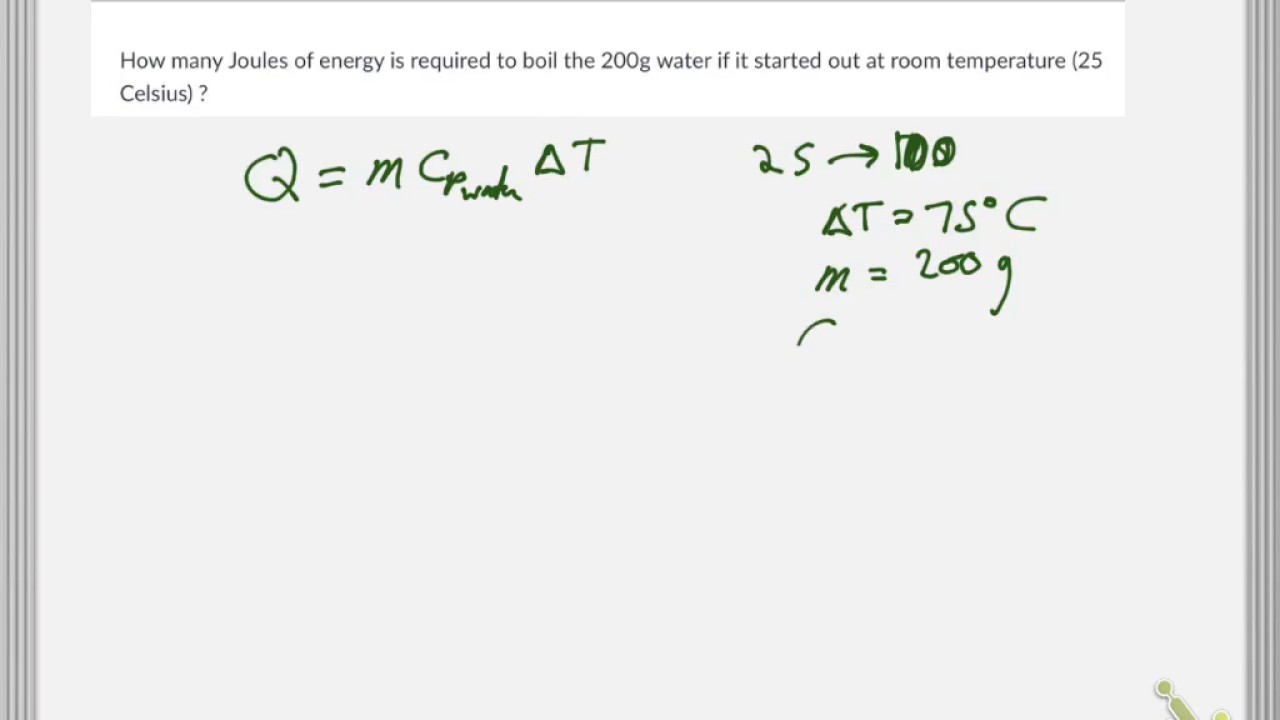The heat energy required to raise the temperature of 2kg of water from 10^0C to 50^0C is (Specific heat capacity of water is 4200 J Kg^0C^-1)
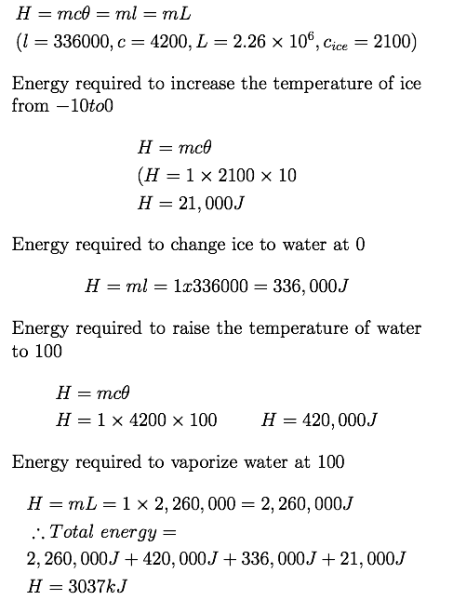
calculate the total energy required to evaporate completely 1kg of ice that is initially at... - Myschool

Question Video: Finding the Amount of Energy Needed to Change the State of Water from Liquid to Gas | Nagwa

How Much Thermal Energy Is Required To Heat Ice Into Steam - Heating Curve Chemistry Problems - YouTube