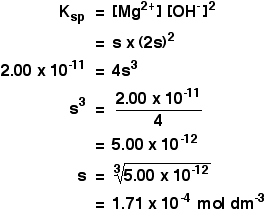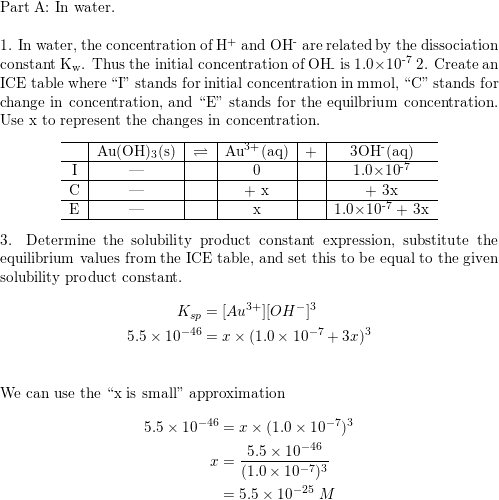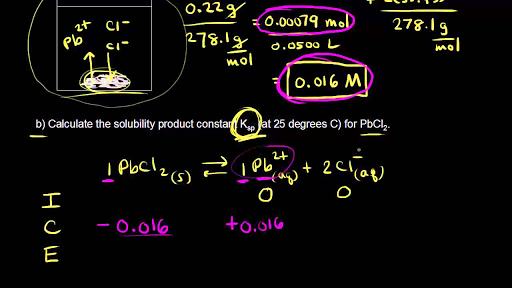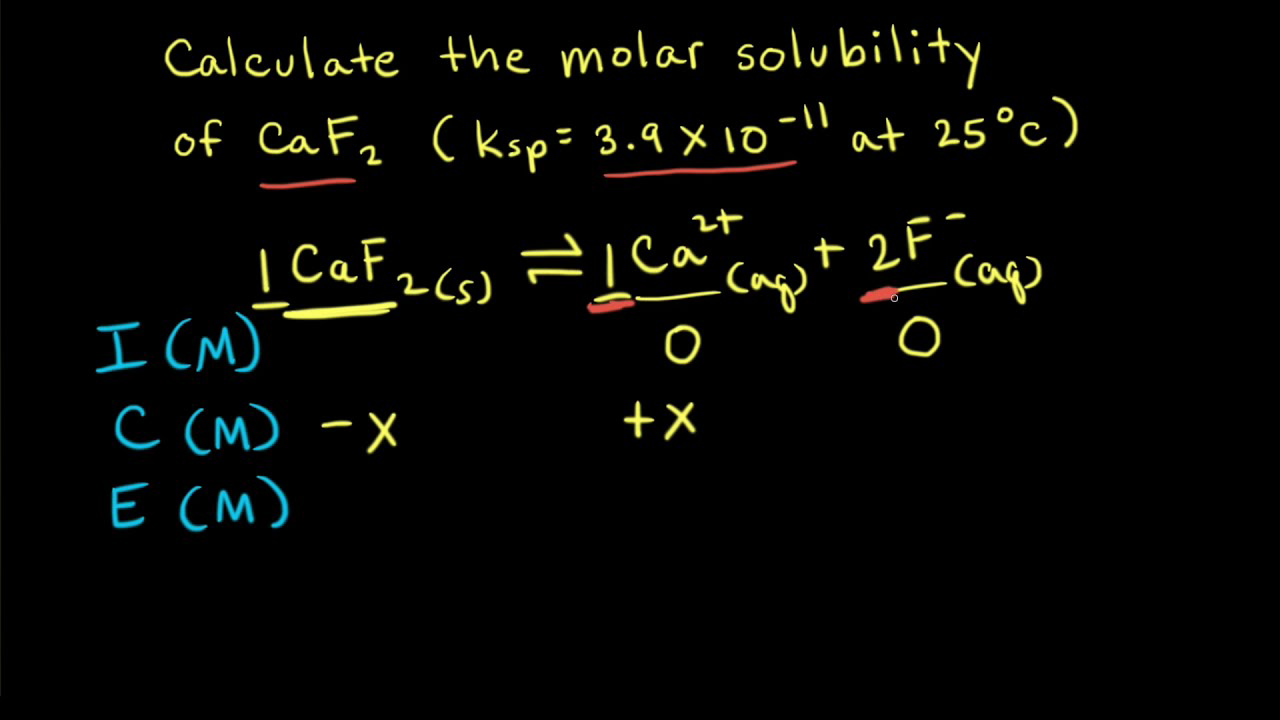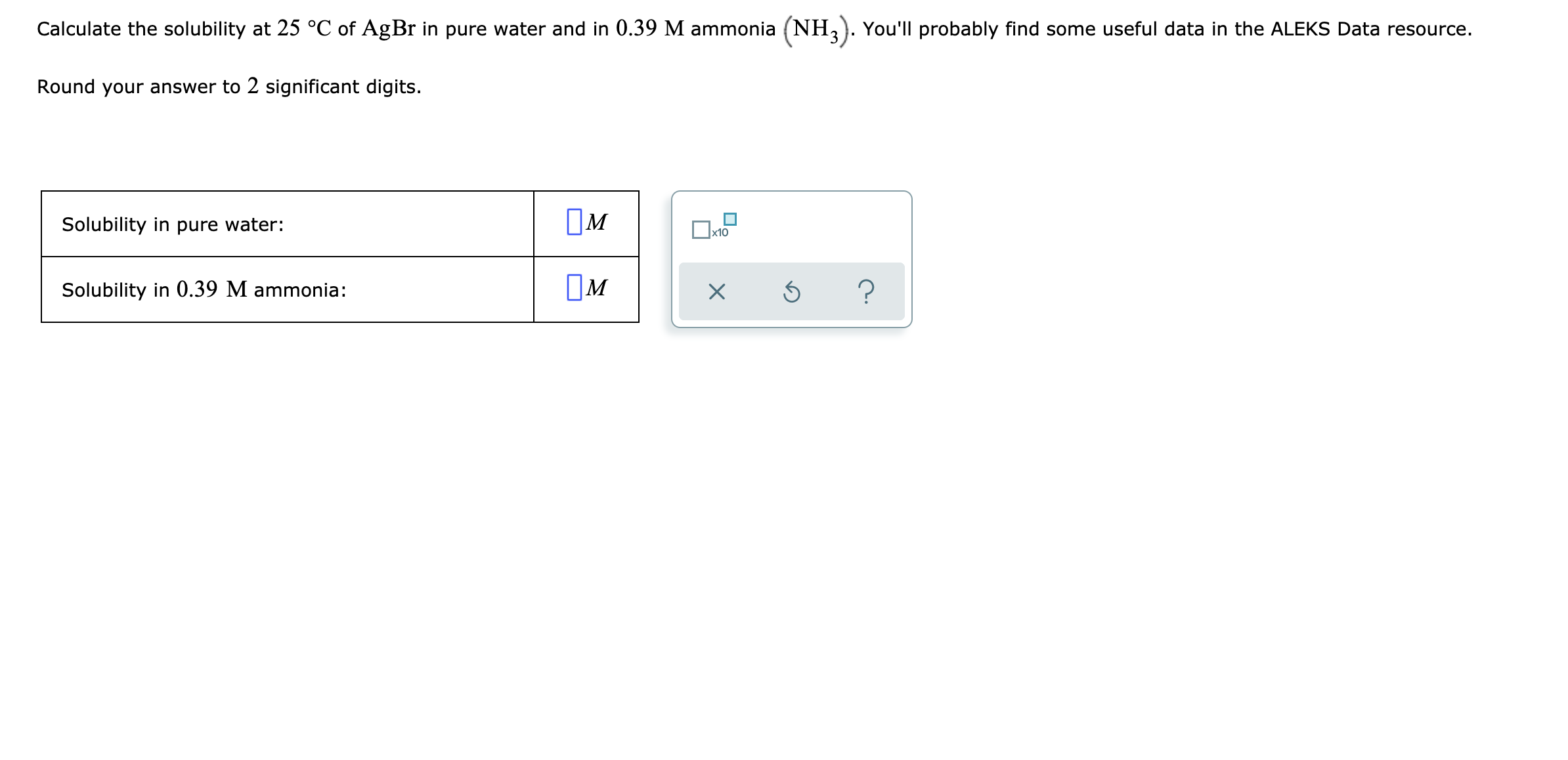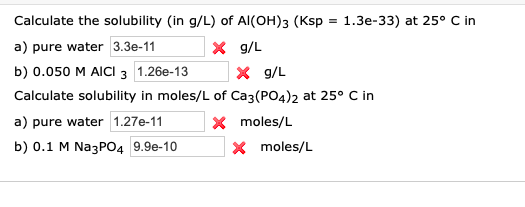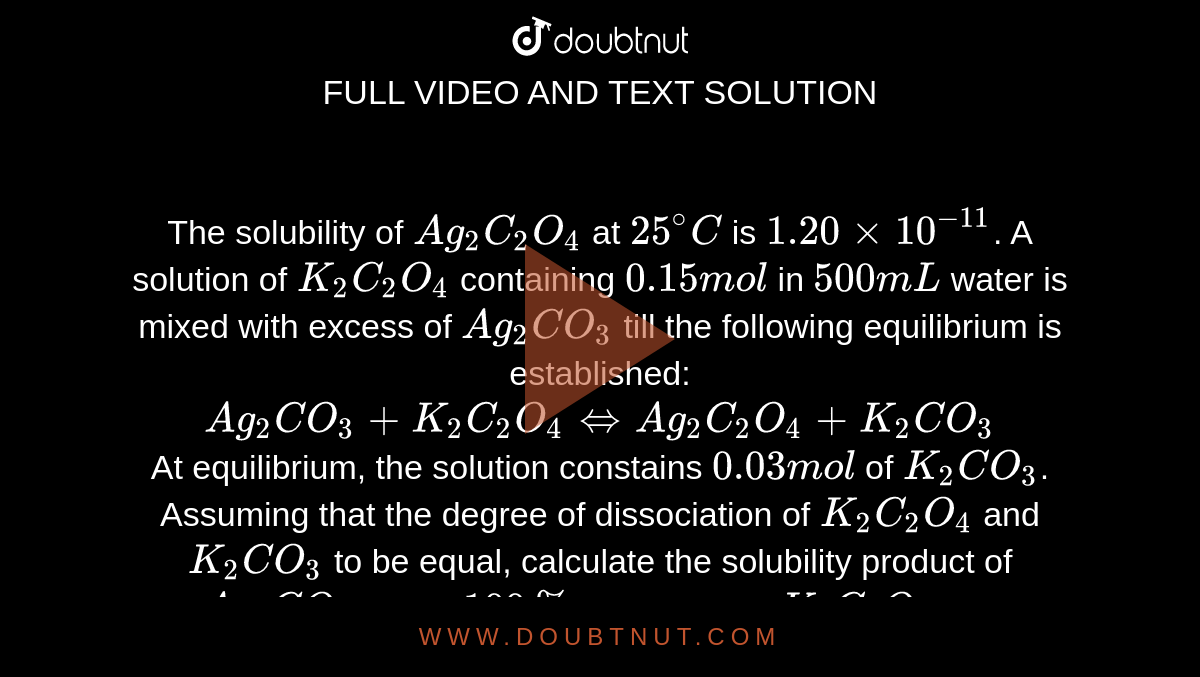
The solubility of Ag(2)C(2)O(4) at 25^(@)C is 1.20 xx 10^(-11). A solution of K(2)C(2)O(4) containing 0.15mol in 500mL water is mixed with excess of Ag(2)CO(3) till the following equilibrium is established: Ag(2)CO(3) +
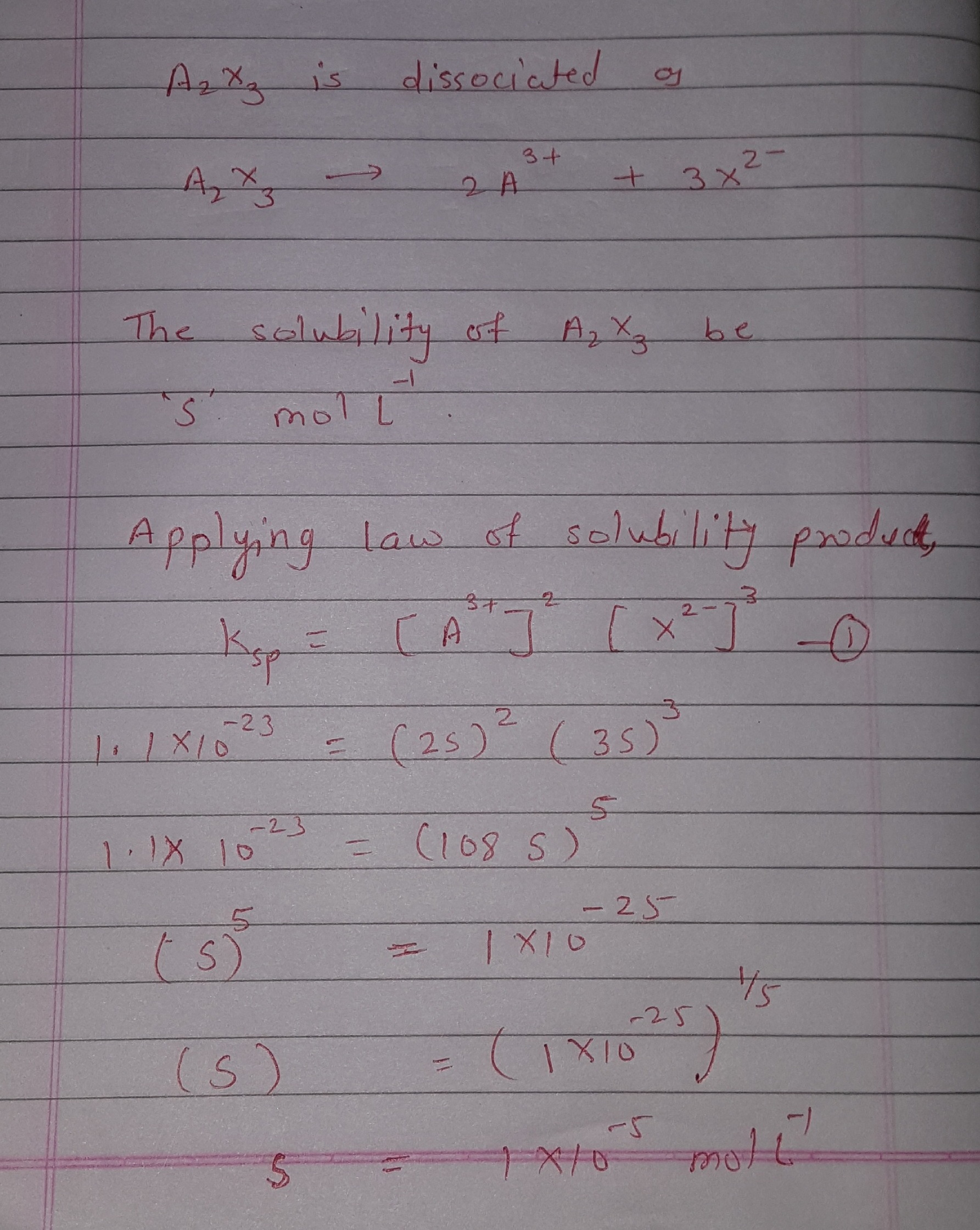
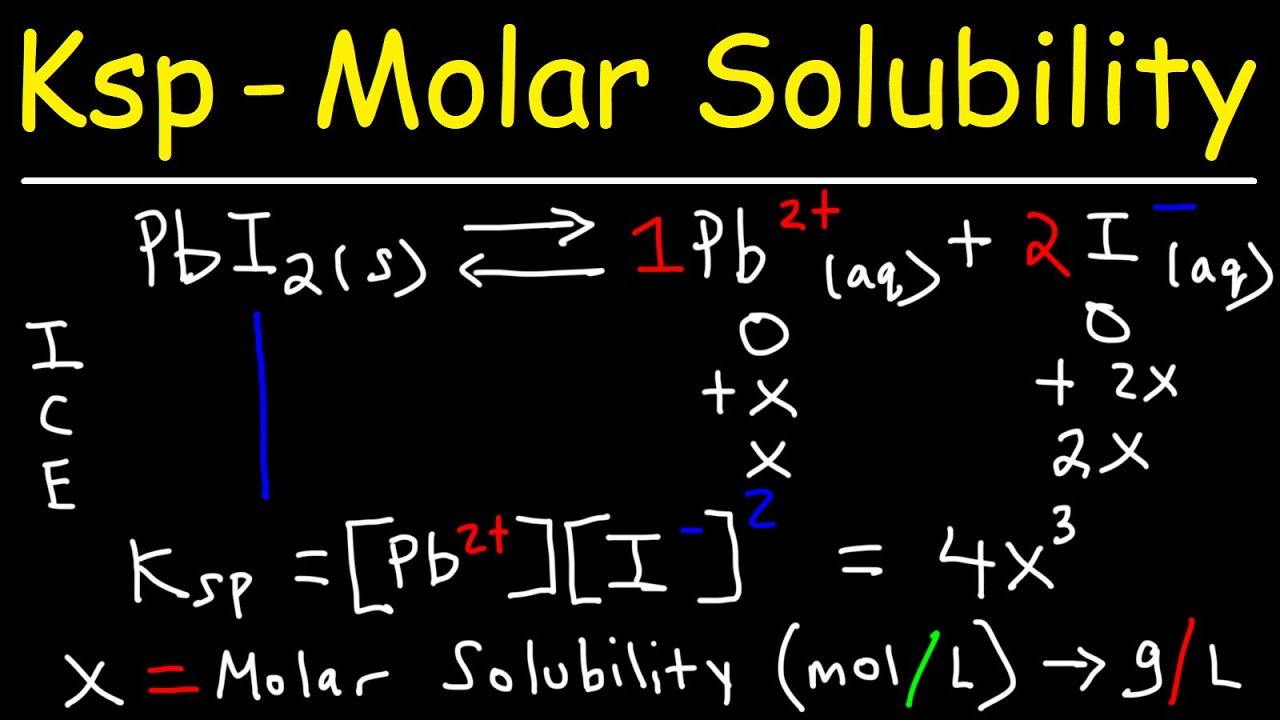
calculate the solubility of a2x3 in pure water assuming that neither kind of ions react with water the solubility product of a2x3 ksp 11 10 power 23 1lo0xyy -Chemistry - TopperLearning.com
![SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH) SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH)](https://cdn.numerade.com/ask_images/7d7ca9e3ac22444fbaeef36ffd903405.jpg)
SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH)