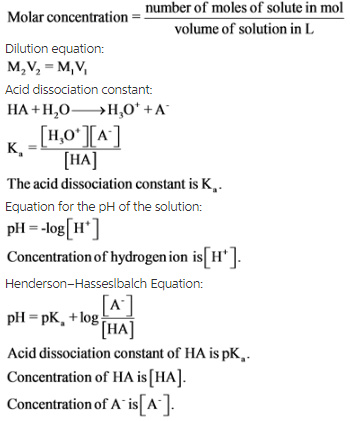
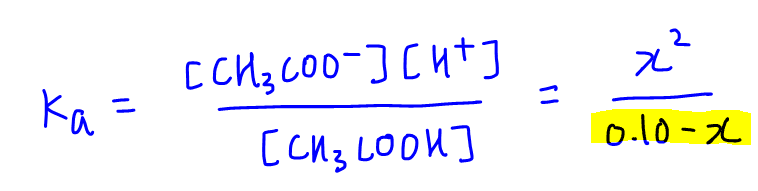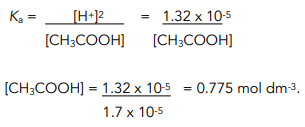A 0.21 M solution of chloroacetic acid, ClCH2CO2H, has a pH of 1.79. Calculate Ka for the acid. | Homework.Study.com
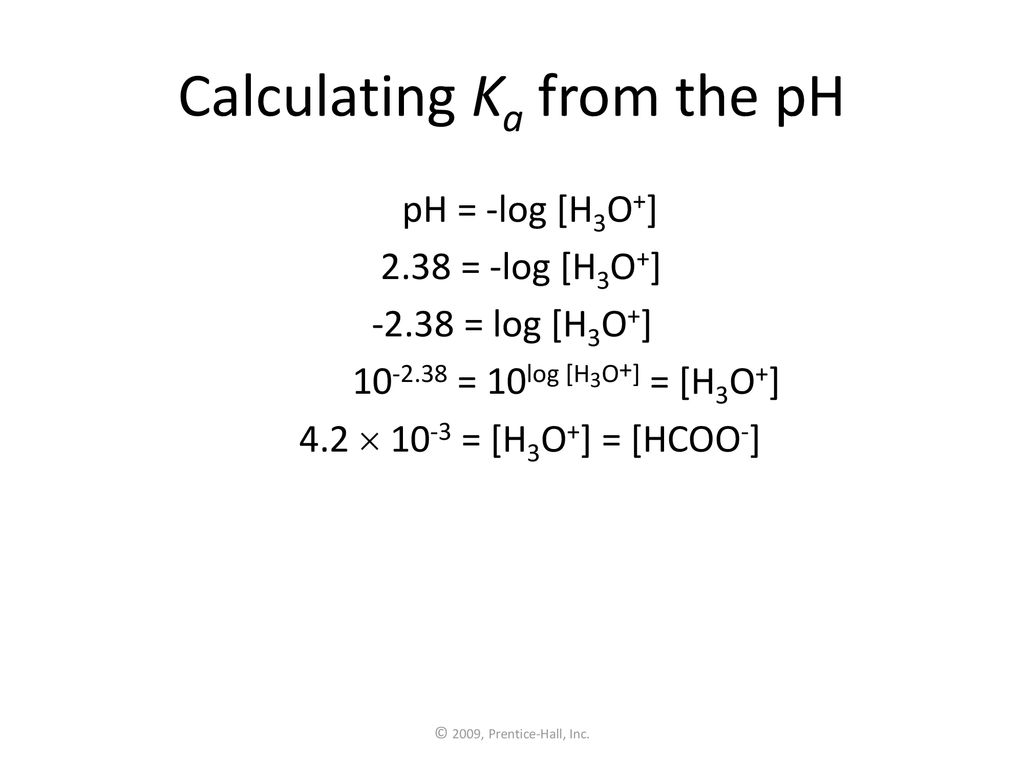
![SOLVED:Calculating [H^+] and p Ka from the pH of a Solution of Weak Acid The pH of a 0.02 M solution of an acid was measured at 4.6. a. What is the [ SOLVED:Calculating [H^+] and p Ka from the pH of a Solution of Weak Acid The pH of a 0.02 M solution of an acid was measured at 4.6. a. What is the [](https://cdn.numerade.com/previews/12327d24-029f-4354-ad85-c698faee7c3c_large.jpg)
SOLVED:Calculating [H^+] and p Ka from the pH of a Solution of Weak Acid The pH of a 0.02 M solution of an acid was measured at 4.6. a. What is the [

Calculate Ka of acetic acid if its 0.05 M solution has molar conductivity of 7.814 × 10^-4Ω ^-1 m^2 mol^-1 at 25^∘C. Given : Λ m^∘ for CH3COOH = 3.907 × 10^-2Ω ^-1 m^2 mol^-1.
![Calculate Ka of acetic acid from equilibrium concentration given below: [H(3)O^(+)]=[CH(3)COO^(-)]=1.34xx10^(-3)M, [CH(3)COOH]=9.866xx10^(-2)M Calculate Ka of acetic acid from equilibrium concentration given below: [H(3)O^(+)]=[CH(3)COO^(-)]=1.34xx10^(-3)M, [CH(3)COOH]=9.866xx10^(-2)M](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/161343719_web.png)
Calculate Ka of acetic acid from equilibrium concentration given below: [H(3)O^(+)]=[CH(3)COO^(-)]=1.34xx10^(-3)M, [CH(3)COOH]=9.866xx10^(-2)M